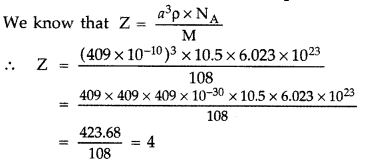Table of Contents
Solid State Important Questions And Answers PDF
Solid State Chemistry Class 12 Important Questions with Answers free pdf solid state questions pdf solid state questions and answers solid state questions for neet pdf solid state question bank with answers solid state previous year questions solid state questions jee solid state pdf solid state class 12 types of solid state solid state ppt solid state imp mcqs solid state physics solid state questions for neet solid state questions for neet pdf download solid state questions for neet pdf solid state questions pdf solid state questions and answers pdf solid state questions for jee mains solid state questions iit jee pdf solid state questions iit jee solid state questions mcq jee mains solid state questions plus two chemistry solid state questions csir net solid state questions class 12 solid state questions jee advanced solid state questions cbse solid state questions neet 2019 solid state questions ncert solid state questions
Question 41.
A metallic element crystallises into a lattice having a pattern of AB AB … and packing of spheres leaves out voids in the lattice. What type of structure is formed by this arrangement?
Answer:
Tetrahedral void is formed in AB AB … pattern. The hexagonal close packing (hep) is formed in this arrangement.
Question 42.
A metallic element crystallises into a lattice having a ABC ABC … pattern and packing of spheres leaves out voids in the lattice. What type of structure is formed by this arrangement?
Answer:
Octahedral voids are formed in ABC ABC … pattern. The cubic close packing (ccp) is formed in this arrangement.
Question 43.
What type of Stoichiometric defect is shown by AgCl?
Answer:
Frenkel defect.
Question 44.
What type of stoichiometric defect is shown by NaCl?
Answer:
Schottky defect is shown by NaCl.
Question 45.
Which ionic compound shows both Frenkel and Schottky defects?
Answer:
Silver bromide (AgBr) shows both Schottky and Frenkel defect.
The Solid State Class 12 Important Questions Short Answer Type – I (SA – 1)
Question 46.
Explain how you can determine the atomic mass of an unknown metal if you know its mass density and the dimensions of unit cell of its crystal.
Answer:
Suppose edge of the unit cell = a pm
Number of atoms present per unit cell = Z
∴ Volume of unit cell = (a pm)3
= (a × 10-10cm)3 = a3 × 10-30 cm3
Density of unit cell = Mass of unit cell Volume of unit cell ……………… (i)
Mass of unit cell = Number of atoms in the unit cell × mass of each atom
= Z × m
Mass of each atom = Atomic mass Avogadro’s no. =MN0
Substituting these values in equation (i), we get
Density of unit cell = Z×Ma3×10−30×N0
If a is in cm, d = Z×Ma3×N0 g/cm3
∴ Molar mass can be calculated as
M = d×a3×N0Z
Question 47.
Calculate the packing efficiency of a metal crystal for a simple cubic lattice.
Answer:
Percentage efficiency of packing of simple cubic lattice = 52.4%.
Question 48.
Define the following terms in relation to crystalline solids :
(i) Unit cell (ii) Coordination number Give one example in each case. (All India) 2011
Answer:
(i) Unit cell : The smallest three dimensional portion of a complete space lattice which when repeated over and again in different directions produces the complete space lattice is called the unit cell.
Example: Cubic unit cell, Hexagonal unit cell etc.
(ii) Coordination number : The number of nearest spheres with which a particular sphere is in contact is called co-ordination number.
Example : Co-ordination number of hexagonal (hep) structures is 12.
Question 49.
The unit cell of an element of atomic mass 108 u and density 10.5 g cm-3 is a cube with edge length, 409 pm. Find the type of unit cell of the crystal. [Given : Avogadro’s constant = 6.023 × 1023 mol-1]
Answer:
So it forms cubic- closed packed (ccp) lattice or fee structure.
Question 50.
Explain the following terms with suitable examples : Ferromagnetism and Ferrimagnetism
Answer:
Ferromagnetic solids : The solids which are strongly attracted by external magnetic field and do not lose their magnetism when the external field is removed are called ferromagnetic solids. The property, thus exhibited, is termed as ferromagnetism.
Example: Fe, Co and Ni show ferromagnetism at room temperature.
Ferrimagnetic solids : The solids which are expected to show large magnetism due to the presence of unpaired electrons but in fact have small net magnetic moment, are called ferrimagnetic solids.
Example : Fe3O4 and ferrites.